The immune system acts as a defense line to protect individuals from different infectious agents such as bacteria, toxins and viruses. A key requirement to fulfill this task is to be able to distinguish ‘foreign agents’ from ‘self’ in healthy individuals, a process that needs to be tightly regulated. When these regulation mechanisms go wrong however, the immune system will attack not only pathogens but the body’s own tissues which can then result in a so-called autoimmune disease (Riedhammer and Weissert, 2015; Mohammadi et al. 2022).
In the 1940s serum derived factors were associated for the first time with autoimmune diseases, and it was shortly after that that these ‘serum factors’ were identified to be autoantibodies derived from the body itself. From this point on, awareness increased that the body was capable of self-damage (Ahsan, 2021). To date more than 80 autoimmune disease are known, often presenting with severe or even fatal symptoms (Rose, 2016).
Autoimmune diseases manifest as chronic diseases; to date there is no cure (https://www.healthdirect.gov.au/autoimmune-diseases). It is possible though to slow down disease progression by treatment which makes early onset treatment a crucial tool to prevent body damage (Rose 2015; Miller 2022). Early diagnosis is therefore indispensable in the fight against the gradual self-damage of the body.
The identification of autoimmune antibodies in patient samples and the discovery that many are specific for a certain type of disease, led to the development of various diagnostic immunoassays to identify the exact nature of the symptoms.
The basic setup principle of such an immunoassay for the detection of autoantibodies is depicted in Figure 1.
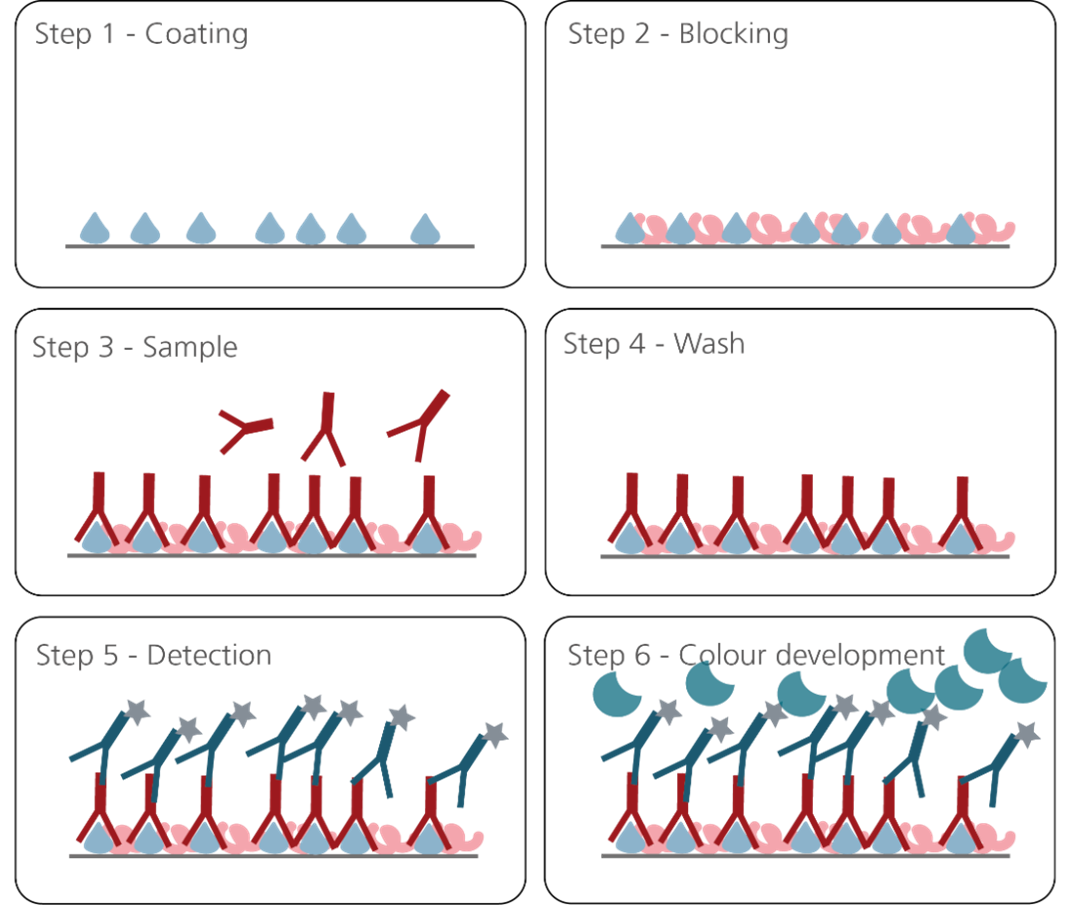
Figure 1.
- Step 1: Coating with antigen
- Step 2: Block remaining binding sites on the solid phase
- Step 3: Add sample with antibody analyte
- Step 4: Wash out excess reagents (separates antibody-bound from free analyte)
- Step 5: add anti-antigen antibody or anti-human IgG secondary antibody conjugated to horseradish peroxidase (HRP) or alkaline phosphatase (AP/ALP) enzyme
- Step 6: Add enzyme substrate: The colour change/amount of light emitted is proportional to the level of target analyte
In such an immunoassay or every similar assay setup used for the detection of autoantibodies the quality of the antigens used are crucial to avoid false-positive or false-negative results. Therefore, one needs to ask the question what the requirements are for an antigen to correctly distinguish healthy samples from patient samples in a variety of assay formats.
An antibody will be able to detect its target when the respective epitope, a small stretch in the antigen the antibody is directed against, is present.
There are typically two types of epitopes: linear and conformational. Linear epitopes need to be exposed and accessible, for conformational epitopes the correct protein folding is usually necessary to enable the binding of the antibody (Goldsby et al., 2003).
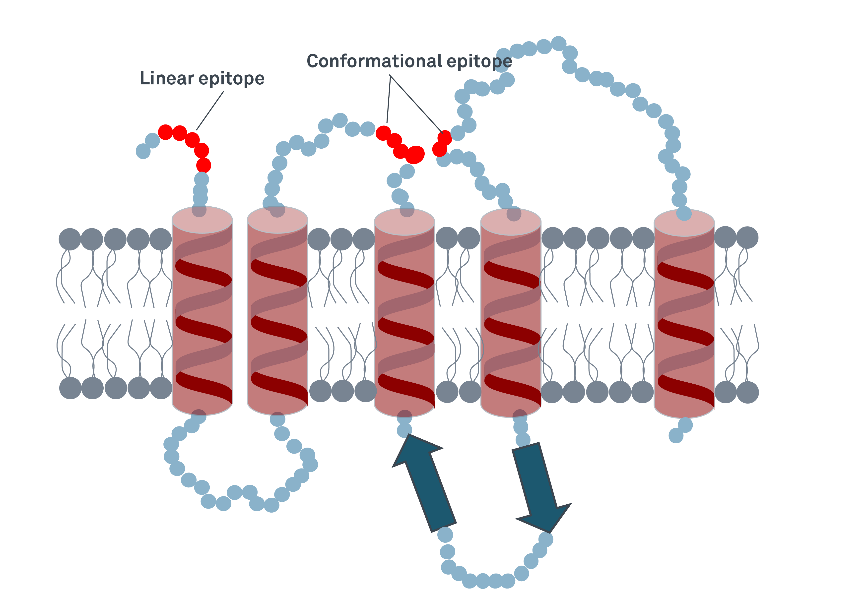
Figure 2: Linear and conformational epitopes in a transmembrane protein. While the linear epitope will potentially be detectable by an antibody in a denatured protein still, detection of the conformational epitope could be impaired when the protein is improperly folded.
When setting up an immunoassay it is therefore important to know that the correctly folded epitopes are present in the antigen and that the respective antibodies can bind.
In many immunoassays native, non-recombinant antigens are therefore used to ensure the most natural conformation one can get. However, the use of native antigens can come with certain disadvantages:
- Depending on the source material continuous supply can be impeded
- The source material often is expensive and subsequently the product derived from the material is as well
- For ethical reasons human source material is often not available and animal tissue is used. However, animal proteins are often not 100% conserved with the human protein sequence and this can result in decreased affinity of the patient autoantibody to the antigen
- The protein of interest could be present in low abundance in the source material and contamination with more abundant proteins could be a problem
- Source material can vary significantly, i.e. when derived from different patient material. This can lead to high lot-to-lot variance which is an issue for test standardization and inter-assay variation.
Under these aspects, are recombinant antigens the better choice when developing an immunoassay? While there is no singular answer to that question, high quality recombinant antigens are often the best solution.
Recombinant antigens can be produced in different expression systems for example and each system comes with specific advantages but also drawbacks (Table 1).
| Expression system | Advantages | Disadvantages |
|---|---|---|
| E.coli cell expression system |
|
|
| Yeast cell expression system |
|
|
| Baculovirus / Insect cell expression system |
|
|
| Mammalian expression system |
|
|
Wherever a specific immunological reactivity with for example patient sera is given, however, recombinant proteins have immense advantages:
- The source material, such as Hek293T cells, CHO cells, Sf9 cells or E. coli is relatively abundant and can be propagated easily in-vitro.
- The protein of interest can be expressed in high amounts and will therefore be present in high percentages compared to the proteins of the expression system.
- The proteins can be tagged to facilitate protein harvesting and purification. Highly pure proteins can therefore be obtained.
- Human protein sequences can be expressed easily with no ethical concerns.
- Due to standardized source material and production processes lot-to-lot variance is minimized which is a prerequisite for immunoassay standardization and low inter-assay variation.
- If required, proteins can be modified to improve handling and to further optimize assay results (please click here to learn more about our optimized tTg antigen)
In summary, one could say that there is no such thing as the perfect expression system. However, when the main requirement, the immunological reactivity, is given, a huge benefit lies in using recombinantly produced antigens.
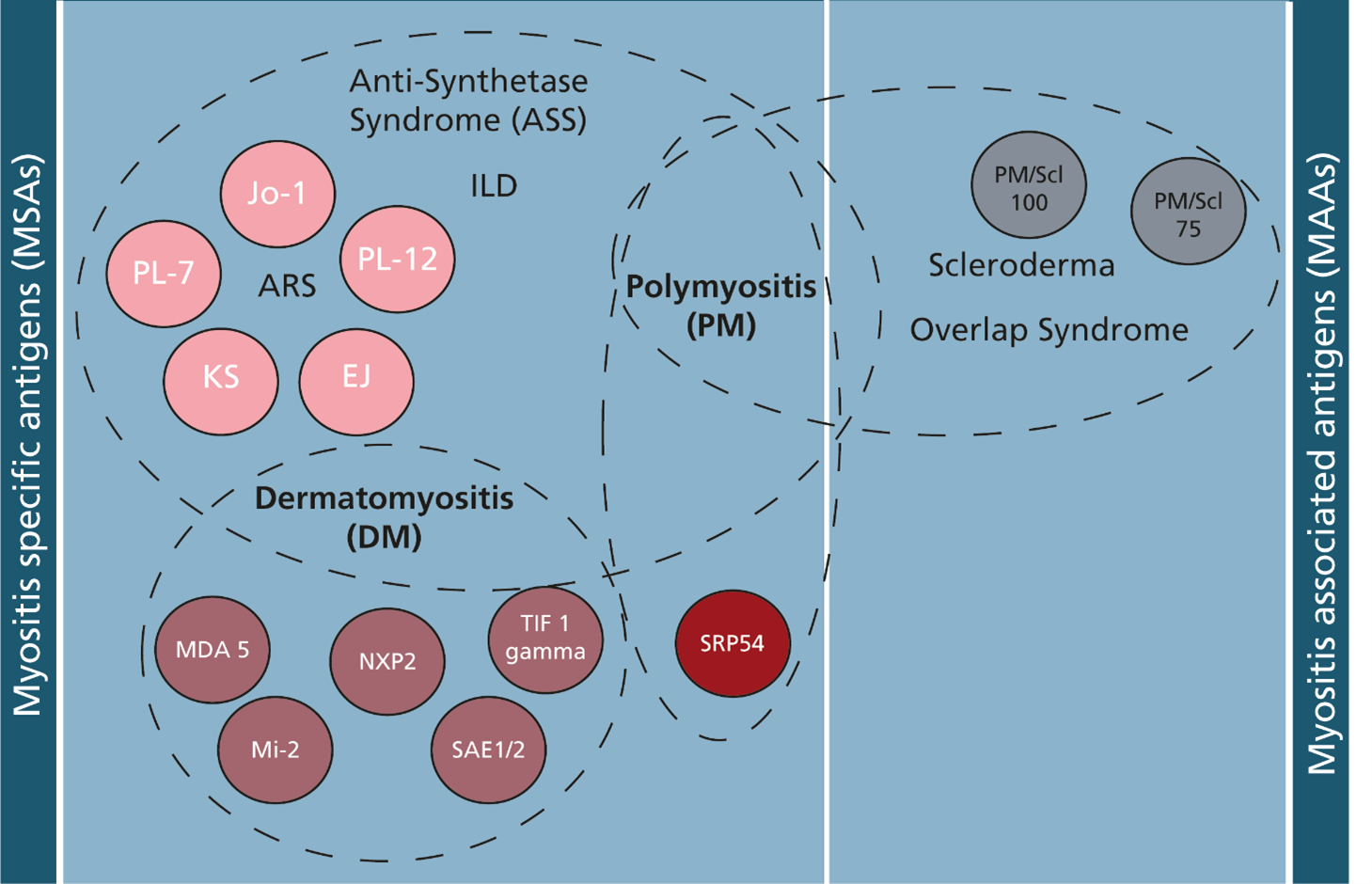
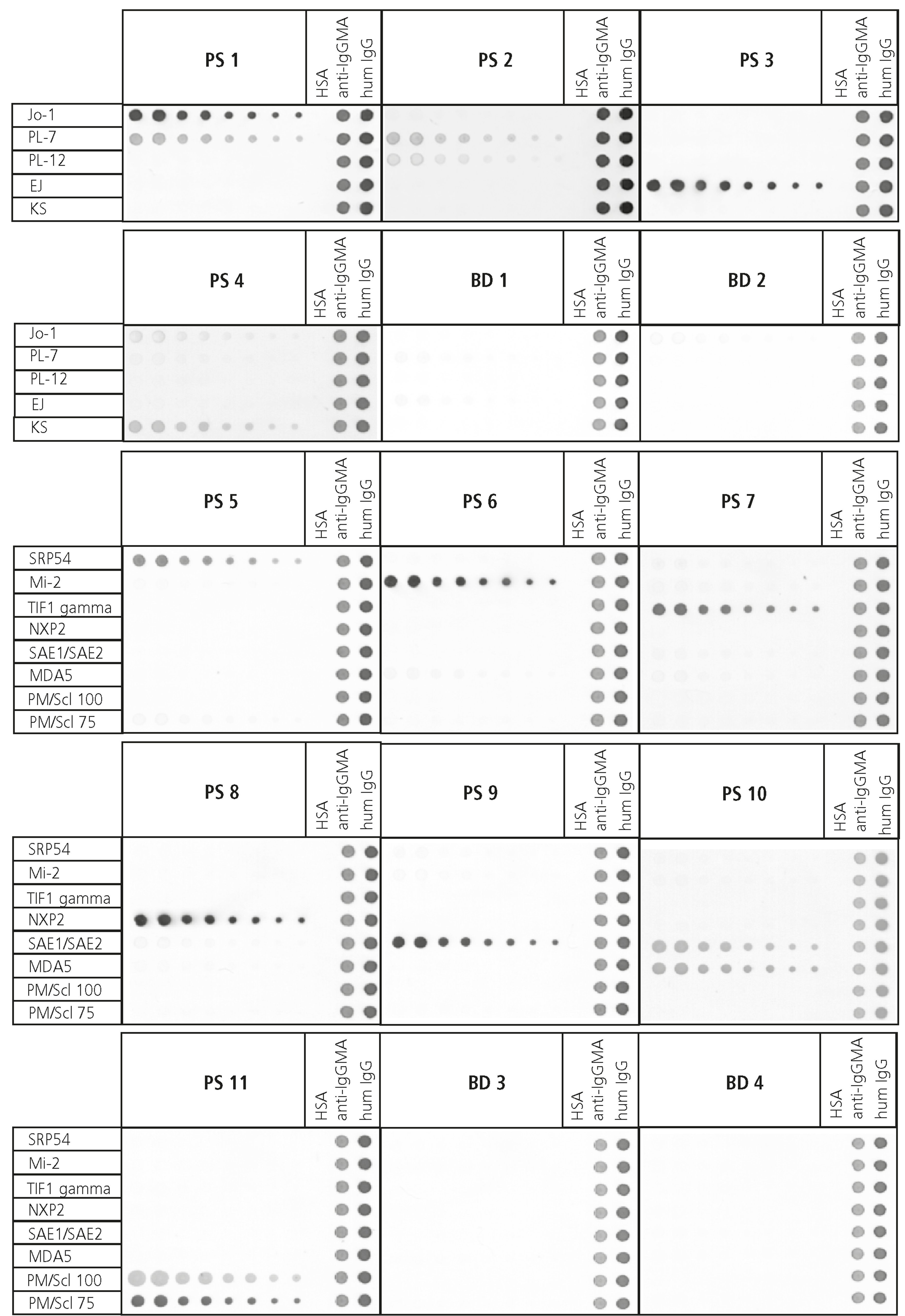
Most of our autoimmune antigens are therefore produced recombinantly and thoroughly tested for immunological reactivity by using healthy and patient samples (Figure 3B). We thereby aim to ensure to provide the best possible quality products to our customers to aid their mission to provide secure diagnosis at an early stage for their patients.





