Plasma Proteins
Plasma Proteins you can trust
BBI provides a wide range of plasma protein products derived from human plasma and serum. The source material is always screened for HIV, Hepatitis B and C prior to use.
Plasma proteins are normally present in relatively high abundance but need separation from other indigenous high abundance proteins such as Albumin and Gamma globulin. A wide range of techniques are used including salt precipitation, dialysis, hollow-fibre and tangential flow filtration, followed by a series of different chromatography techniques including Ion exchange, ligand affinity, hydrophobic interaction, size exclusion and immunoaffinity. The purified proteins are then formulated liquid or lyophilised in the most appropriate stabilising buffer and filtered to 0.2µm to ensure low bioburden.
Products
| Product Name | Code | Type | Applications | |
|---|---|---|---|---|
| Albumin | P140-0 | Antigens/Proteins | Biosensor, Clinical Chemistry, Control Manufacture, ELISA, Life Sciences | Buy now |
| Prealbumin | P171-1 | Antigens/Proteins | Biosensor, Clinical Chemistry, Control Manufacture, ELISA, Life Sciences | Buy now |
| Transferrin Receptor | P185-9 | Antigens/Proteins | Biosensor, Clinical Chemistry, Control Manufacture, ELISA, Life Sciences | Buy now |
| Thyroxine Binding Globulin >70% | P125-2 | Antigens/Proteins | Biosensor, Clinical Chemistry, Control Manufacture, ELISA, Life Sciences | Buy now |
| Thyroxine Binding Globulin >98% | P125-1 | Antigens/Proteins | Biosensor, Clinical Chemistry, Control Manufacture, ELISA, Life Sciences | Buy now |
| Alpha-1-Antichymotrypsin | P159-5 | Antigens/Proteins | Biosensor, Clinical Chemistry, Control Manufacture, ELISA, Life Sciences | Buy now |
| Alpha-1-antitrypsin | P165-5 | Antigens/Proteins | Biosensor, Clinical Chemistry, Control Manufacture, ELISA, Life Sciences | Buy now |
Brochure
Products & Services
Inside this catalog you’ll find one of the industry’s broadest collections of enzymes, antibodies, antigens, and more – meticulously developed to power world-class diagnostics and keep your pipeline moving.
Download
Brochure
Reagents Range
Discover our collection of reagents covering a comprehensive range of biomarkers and applications for the IVD market, life sciences, cell culture and beyond.
Download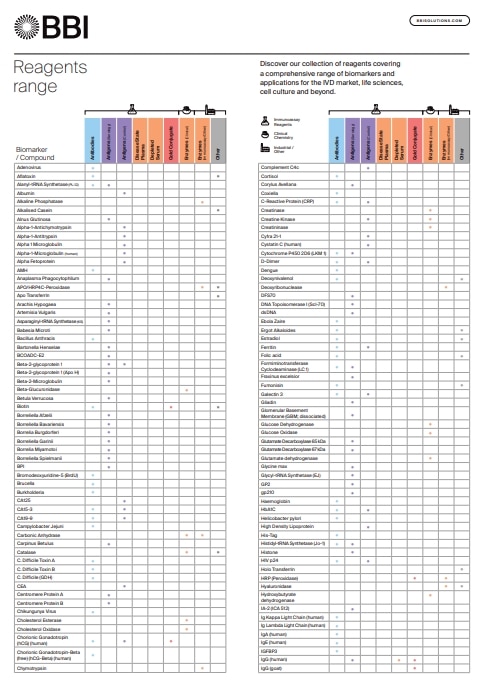

What are antigens and how are they used?
BBI antigens are purified human proteins—either native or recombinant—that serve as critical reagents for making in-vitro-diagnostic (IVD) calibrators and quality-control materials; in some cases they also provide specific biological functionality for research or cell-based assays.
What is the source material for your antigens?
Depending on the product, antigens are produced recombinantly in prokaryotic or eukaryotic systems, purified from consented human tissues or body fluids, or harvested from well-characterised human cell lines.
Where are the antigens manufactured and purified?
Manufacturing takes place at BBI’s ISO 13485-certified facilities in Crumlin, UK, and Freiburg, Germany, both of which operate strict quality-management systems and traceable batch records.
How does BBI guarantee lot-to-lot consistency?
Every production lot follows documented SOPs, is subjected to biochemical and immunological testing, and must meet tight release specifications before it leaves QC, ensuring reproducible performance in your assay.
How should I handle the material when it arrives?
Store at the temperature specified on the CoA.
How can I obtain pricing?
Pricing is volume-dependent; you can check our online shop or request a formal quotation from your account manager or contact customer services.
How do I check stock availability or secure future supply?
Your account manager can provide current inventory levels, arrange reservations, and help integrate your six-month forecasts into our production planning. You can also contact us or use our online shop if you don’t have an account manager yet.
What is the process if I have a complaint or quality concern?
All complaints should be sent to Complaints@bbisolutions.com. All issues are investigated under BBI’s ISO 13485 quality-management system, and we will work with you to reach a satisfactory resolution.
Request a sample
Product purity and testing
Products are characterised prior to fill to determine the recovery and purity of the protein using a range of analytical techniques. Immunological recovery is tested using specific immunoassays standardised against reference preparations. Where practical, BBI will offer to define the recovery on a specific IVD platform immunoassay system such as the Siemens ProSpec system. Techniques such as Coomassie stained SDS-PAGE are used to test and verify the product purity.
After the products have been filled and formulated, they are retested by our independent Quality Control laboratory to verify the analytical results and define the biological integrity. BBI has a wide range of plasma proteins which are supplied as standard products from stock, plus there are a number of products that we make to order.
As we are the manufacturer, we can provide the most cost-effective solution for our clients. Most plasma protein products are supplied as highly purified or part-purified preparations. Some of our clients require specific high purity proteins and, in some cases, we offer our ultra-pure grade for use as immunogens. For these materials trace impurities are removed by immunoaffinity absorption using an affinity resin coupled to antiserum to normal human serum. Testing of the ultra-pure grades includes assessment using immunoelectrophoretic techniques.

BBI's Plasma Proteins
BBI have been manufacturing a range of purified plasma protein products for over 40 years. The plasma proteins are sourced from reliable and highly regulated plasma sources; all source material is screened for safety using the latest methods. The products are made to order or available from stock using processes defined by specific batch records. We endeavour to always supply material to our customers’ timelines but rely on our clients to provide forecasts and estimates of usage.
Materials are prepared using a range of purification technologies, some of these unique to BBI. Products are available as either highly purified or part-purified grades, are formulated to optimise shelf-life and provide material in the most convenient storage format. Analytical testing is performed to demonstrate the product purity and recovery using immunological and/or biochemical protein assays.
Contact Us
BBI's Capabilities
Specialists

We have extensive experience in sourcing raw materials worldwide
Salt/solvent fractionation

Batch sizes of up to 5,000 litres
Chromatography

Ion exchange chromatography, affinity chromatography, size exclusion chromatography, hydrophobic interaction chromatography
Crystallisation

Highly effective protein purification using solvent and aqueous media
Ultrafiltration

Volumes of 2,500 - 30,000 litres
Freeze drying
Capability of 49 - 420 litres per cycle
Quality control

In-process analysis and monitoring, development of assay/test procedures
Product handling/sampling
We offer special packing according to customer specifications